Learn About Wolfram Syndrome
and ER Stress Diseases
Understand Ongoing Research for Wolfram Syndrome and ER Stress Diseases
Stop Disease Progression
Protect and Regrow Remaining Tissue
Replace and Repair Pathogenic Genes
Stop Disease Progression
Protect and Regrow Remaining Tissue
Replace and Repair Pathogenic Genes
Support Research and Treatments for
Wolfram Syndrome,
Diabetes, and Neurodegenerative Diseases
We have established The Unravel Wolfram Syndrome Fund through Washington University in St. Louis School of Medicine to provide financial support for Dr. Fumi Urano’s research group as they work to secure new treatments and ultimately a cure for Wolfram syndrome.
|
What is Wolfram Syndrome?
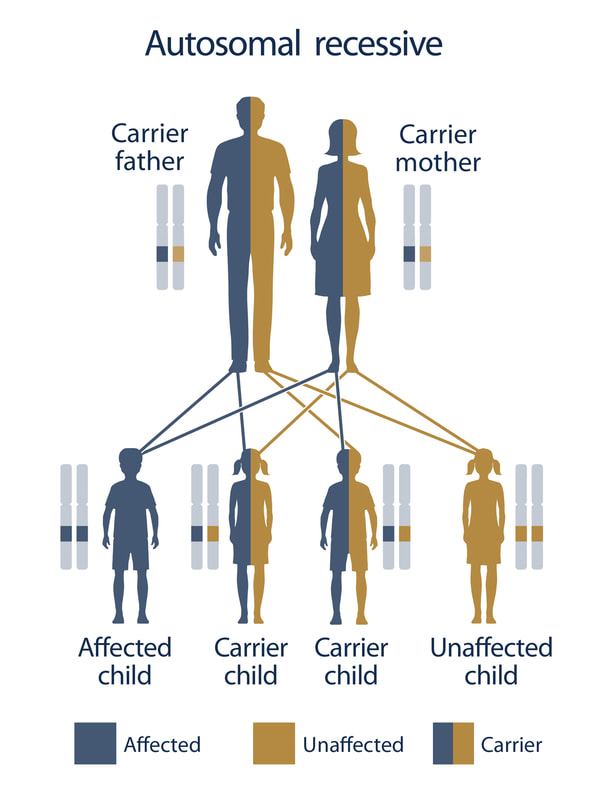
Wolfram syndrome (WS) is an ultra-rare genetic disease that occurs in approximately 1/500,000 people. The typical presentation of WS is insulin dependent diabetes by age 5 to 6, optic nerve atrophy with profound vision impairment or blindness by age 15 to 20, and other medical issues including, but not limited to, diabetes insipidus, deafness, and neurodegeneration.
Death occurs by age 30 in approximately 50% of people with classic Wolfram syndrome. Some mutations in WFS1, the gene that causes WS, can cause a milder form of this disease. Mutations in the CISD2 gene can also cause Wolfram syndrome. Wolfram syndrome is an autosomal recessive disease. To be diagnosed with WS, an individual must have a mutation in both of their copies of the WFS1 gene, that codes for wolframin protein, or both copies of the CISD2 gene. Dominant mutations in WFS1 can cause WFS1-related disorder which results in sensorineural low frequency hearing loss, hearing loss and optic nerve atrophy, or cataracts. This dominant form of disease is distinct and less severe than that of patients with recessive Wolfram syndrome (De Franco et al., 2017). Single mutations in WFS1 can also cause a rare, autosomal dominant syndrome characterized by neonatal diabetes, congenital cataracts, sensorineural deafness, hypotonia, intellectual disability, and development delay. |
Who Has Wolfram Syndrome?
Increasing evidence indicates that Wolfram syndrome can present with varying degrees of severity. Depending on an affected individual's specific mutations, the resulting symptoms, or phenotype, may be milder than the classic disease. Specific mutations may be far more prevalent in certain populations than originally determined, and may result in more frequent adult onset type 1 diabetes as well as an increased risk for type 2 Diabetes.
In May 2016 at age 11 our child was diagnosed with Wolfram syndrome and was not insulin dependent until age 14. This means that the unique combination of recessive mutations present in our family likely has left our child with some functional wolframin protein. With tremendous help and guidance from Dr. Fumi Urano at Washington University in St. Louis, we hope to prolong pancreas function and slow the course of disease. By helping our child, learning more about WS, and supporting Dr. Urano’s research, we hope to do everything we can for people with Wolfram syndrome and their families. |
How Do the Symptoms of Wolfram Syndrome Develop?
|
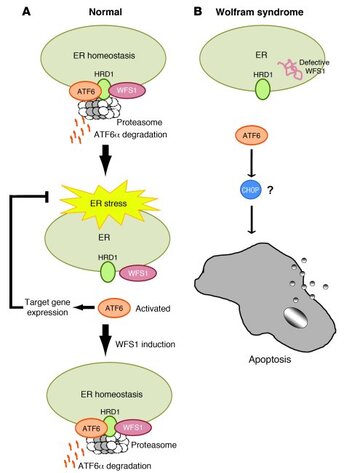
The symptoms of WS are caused by dysfunctional wolframin protein, the protein encoded by the WFS-1 gene. In WS the endoplasmic reticulum (ER), a protein maker for the cell, is not able to do its job well because it lacks functional wolframin protein. Wolframin protein helps fold other proteins properly and maintain proper calcium levels in the ER. Without properly functioning wolframin protein, misfolded proteins accumulate, calcium leaks into the body of the cell, and ultimately the cell dies. The cells most affected in WS are insulin producing beta cells and neurons. Of great interest in WS is the realization that there appear to be 3 levels at which cells are affected. At level 1 cells are still functional. At level 2 cells are dormant and thus still alive, though not functioning properly. At level 3 cells have gone through apoptosis and have died. The cells existing at level 2 have the potential to be revived and thus function restored and protected in patients with Wolfram syndrome. |
In normal cells, pathways that involve wolframin protein are activated to rescue the cell when ER stress occurs. In Wolfram syndrome wolframin is unable to help rescue the cell, and apoptosis (cell death) ultimately occurs.
|
Why Does Wolfram Syndrome Matter?
|
As a family we care about Wolfram syndrome (WS) because our child has WS. However, given that the study of Wolfram syndrome may hold the key to treating millions of other people, WS should matter to all of us.
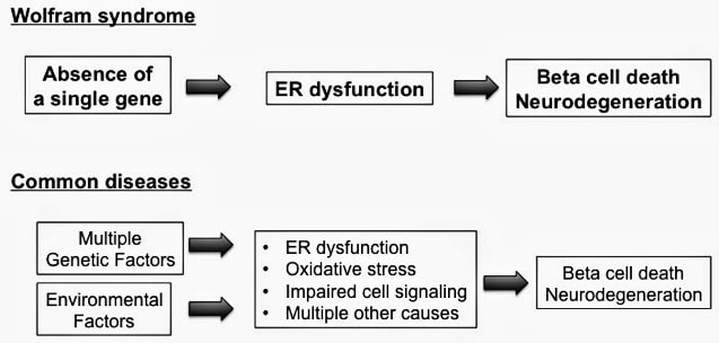
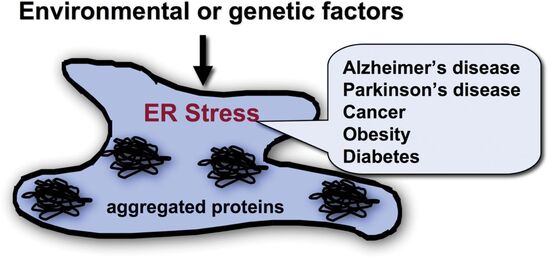
Increasing evidence indicates that ER stress, and ultimately cell death, plays a significant role in common forms of diabetes as well as neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and ALS. WS offers an exquisite example of the role of endoplasmic reticulum (ER) stress in disease. WS is more likely to reveal the mechanisms of ER stress-mediated cell death than other common conditions in which multiple factors typically interact to produce the disease. Thus WS represents an ideal model to help determine the underlying causes of cell death in ER stress-mediated diseases. Originally thought to be a 1/500,000 disease, there is new evidence that mutations of WFS1 occur with far greater frequency in certain populations. This results in increased incidence and prevalence of milder WS, presenting as Type 1 diabetes or optic nerve atrophy. The study of Wolfram syndrome may lead to a breakthrough for treatments of not only Wolfram syndrome, but also more common diseases such as type 1 diabetes, type 2 diabetes, and neurodegenerative diseases. |
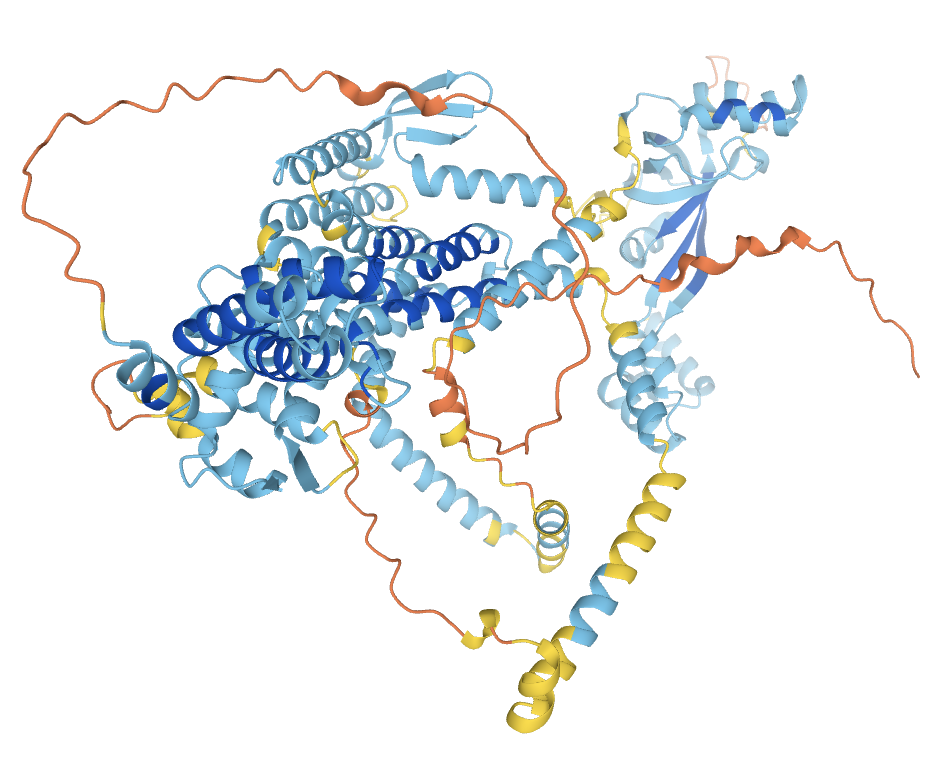
AI model of Wolframin, Courtesy of AlphaFold/DeepMind
|
How Can We Help?
|
Dr. Fumihiko Urano at Washington University in St. Louis School of Medicine is a hero to our family as an international WS expert. Along with his research team, Dr. Urano is tirelessly researching Wolfram syndrome to find a treatment and hopefully a cure for this rare disease. WS may well provide the key to treating millions of people with diabetes, Parkinson’s disease, Alzheimer’s disease, ALS, and other neurodegenerative diseases. As a result of Dr. Urano’s efforts, treatments for WS are within our grasp. For more information please see Dr. Urano's blog and clinical trials.
A treatment is in clinical trial now and other treatments are being evaluated for clinical trial in the near future. More funding is needed to complete these studies. We have established The Unravel Wolfram Syndrome Fund through Washington University in St. Louis School of Medicine to provide financial support for Dr. Urano’s research group. Although we feel an urgency to help our daughter who has Wolfram spectrum, there are many other courageous people with Wolfram syndrome, and millions with other forms of diabetes and neurodegenerative disease for whom there is an even greater urgency. 100% of your donation will go directly to Dr. Urano’s lab and research group to help get the treatments from the lab to the people who desperately need them. |
Please click below and you will be redirected to the Washington University in St. Louis donation page. Please enter your donation amount next to the box labeled
The Unravel Wolfram Syndrome Fund.
(Sections other than "Personal Information" do not need to be completed.)
Your donation is tax deductible.
Our family and many, many others thank you for your support!
For more information about making your donation please call 314-935-9715
To contribute by mail please mail your donation to:
The Unravel Wolfram Syndrome Fund
attn: Rachel Hartmann
Washington University in St. Louis
Campus Box 1247
7425 Forsyth Blvd.
suite 2100
St. Louis, MO. 63105-2161
To contribute by mail please mail your donation to:
The Unravel Wolfram Syndrome Fund
attn: Rachel Hartmann
Washington University in St. Louis
Campus Box 1247
7425 Forsyth Blvd.
suite 2100
St. Louis, MO. 63105-2161